
|
The unintended immune consequences of HIV treatments
From the Hladik Lab, Vaccine and Infectious Disease Division
Science Spotlight
OCTOBER 19, 2020 • BY B TRAXINGER
With lifelong adherence, current antiretroviral therapies (ART) reliably prevent the progression to AIDS in persons living with HIV (PLWH). However, PLWH experience a higher incidence of non-AIDS related illness and death, often defined by chronic, low-level immune activation that persists in the absence of detectable HIV viremia. Despite the benefits of ART, this treatment could contribute to the ongoing immune dysregulation found in PLWH.
Although the nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC), known together as Truvada, are two effective and standard components of ART, their effects on immune dysregulation have not been adequately studied. Sean Hughes, along with colleagues from the Hladik lab in the Vaccine and Infectious Disease Division, hypothesized that Truvada might cause changes in the gastrointestinal (GI) tract, a known site of chronic immune activation in PLWH and the tissue site which harbors the highest HIV load. To test this, they measured gene expression in the GI, female reproductive tract, and blood of HIV-negative people taking Truvada and published their findings in Cell Reports Medicine.
Using tissue biopsies and blood samples from HIV-uninfected individuals before and during a course of oral TDF and FTC given in three clinical trials, Hughes and colleagues assessed gene expression via microarray. They found 13 differentially expressed genes in the GI tissue (rectal and duodenal) from the Truvada treatment group compared to pre-treatment. Although Truvada treatment induced the expression of relatively few genes, nearly all were related to type I and type III interferons, a class of cytokines that activate the immune system in response to pathogens. This suggested that oral Truvada may elicit a previously unappreciated immune activation unrelated to HIV infection. “We only studied Truvada use in HIV-uninfected people, so we know the effects we observed were only due to the drug itself, not to HIV infection,” Hughes explained.
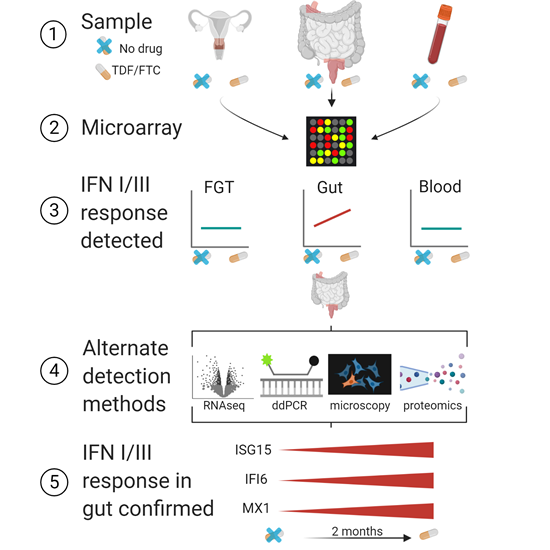
Graphical abstract of study workflow.
Figure from publication, provided by Claire Levy and Florian Hladik. Interestingly, neither reproductive tract tissue nor blood expressed increased interferon signatures in response to Truvada, suggesting that these therapies have a tissue-specific effect. This finding may seem surprising, but Hughes explained how this data complements previous research. “Truvada is more effective at preventing rectal HIV infection than vaginal HIV infection. We observed increases in these antiviral genes in the rectum, but not in the vagina. That anatomical difference in gene expression might explain why Truvada prevents rectal infection better than vaginal infection,” he said.
To confirm the microarray findings, the authors performed RNA-sequencing on the pre- and post-treatment rectal samples. Although only one gene that was found by microarray to be overexpressed in the treated samples was also significantly differentially expressed via RNA-sequencing, the fold expression change and direction of the 13 original interferon-related hits was consistent between microarray and RNA-sequencing, corroborating the trend of increased immune activation. Additionally, the authors used mass spectrometry of rectal tissue to confirm five of these interferon-related hits at the protein level.
Finally, the authors used digital droplet PCR (ddPCR) to amplify and quantify the expression of three selected interferon-related genes in samples from before and after oral Truvada. In accordance with their previous findings, Hughes and colleagues confirmed that treatment increases type I and type III interferon-related genes in the rectum and duodenum, but not in the female reproductive tract or blood. Additionally, immunofluorescence microscopy revealed an increased number of cells expressing the interferon-stimulated gene ISG15 in the rectal and duodenal tissue during ART treatment compared to pre-treatment, including expression on microfold (M) cells, a subset of intestinal epithelial cells with specialized immune activity.
This study highlights a paradox in ART, where “Truvada is extremely effective at preventing HIV replication” but “also changes human gene expression,” Hughes explained. While “increased expression of type I/III interferon genes could make Truvada better at preventing and treating HIV infection,” he explained that chronically elevated expression of interferon-related genes could “also have negative consequences. For example, chronic inflammation in the gut is common among people living with HIV, but we don’t know why. Increased type I/III interferon gene expression could contribute to chronic inflammation, as well as other inflammation-related conditions that are common in people living with HIV, such as cardiovascular disease. In addition, these effects could interfere with finding an HIV cure, if they make HIV-infected cells more likely to survive or even cause them to proliferate.”
--------------------------------------------------------------------------------------------------------------
Hughes SM, Levy CN, Calienes FL, Stekler JD, Pandey U, Vojtech L, Verard AR, Birse K, Noel-Romas L, Richardson B, Golden JB, Cartwright M, Collier AC, Stevens CE, Curlin ME, Holtz TH, Mugo N, Irungu E, Katabira E, Muwonge T, Lama JR, Baeten JM, Burgener A, Lingappa JR, McElrath MJ, Mackelprang R, McGowan I, Cranston RD, Cameron MJ, Hladik F. 2020. Treatment with Commonly Used Antiretroviral Drugs Induces a Type I/III Interferon Signature in the Gut in the Absence of HIV Infection. Cell Reports Medicine. doi: 10.1016/j.xcrm.2020.100096
Contact
RED HUTCH
Phone: 206.667.2210
Email: media@fredhutch.org
Source: FRED HUTCH
Reproduced with permission - FRED HUTCH"
FRED HUTCH
For more HIV and AIDS News visit...
Positively Positive - Living with HIV/AIDS:
HIV/AIDS News |